Pipeline
Pipeline
- Home
- Pipeline
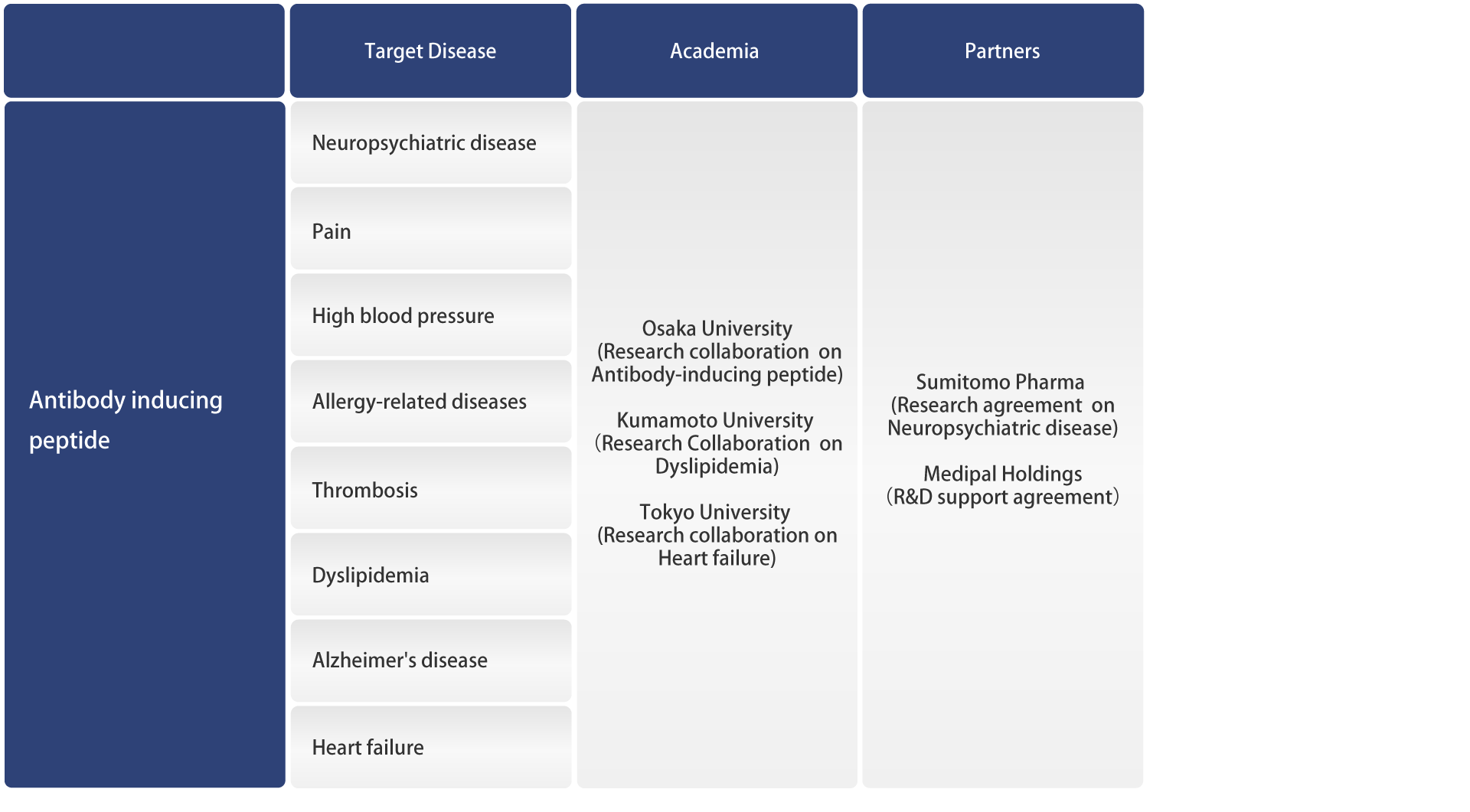
Pipelines
FunPep is a biotech spin out from the Osaka University Graduate School of Medicine focused on various functional peptides to develop a wide range of products from pharmaceuticals to cosmetics.
Development Pipeline
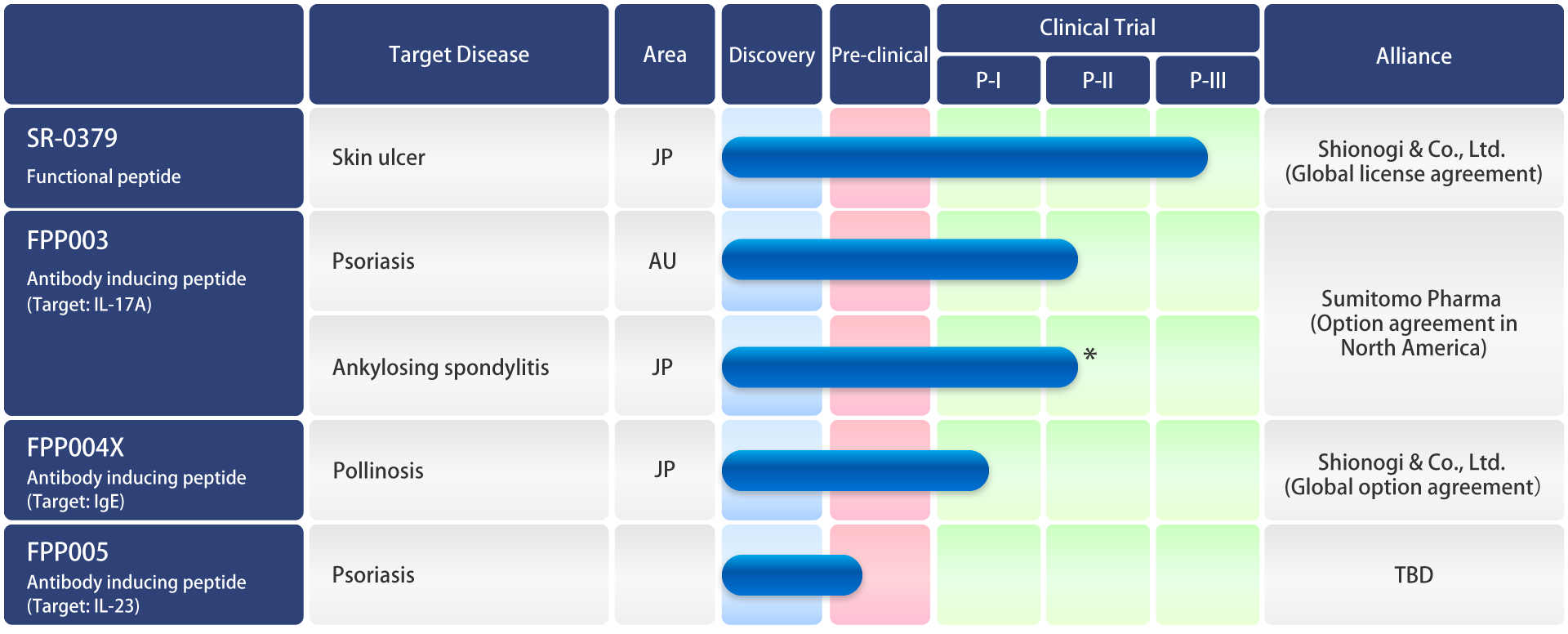
* Investigator initiated trial
Discovery Research